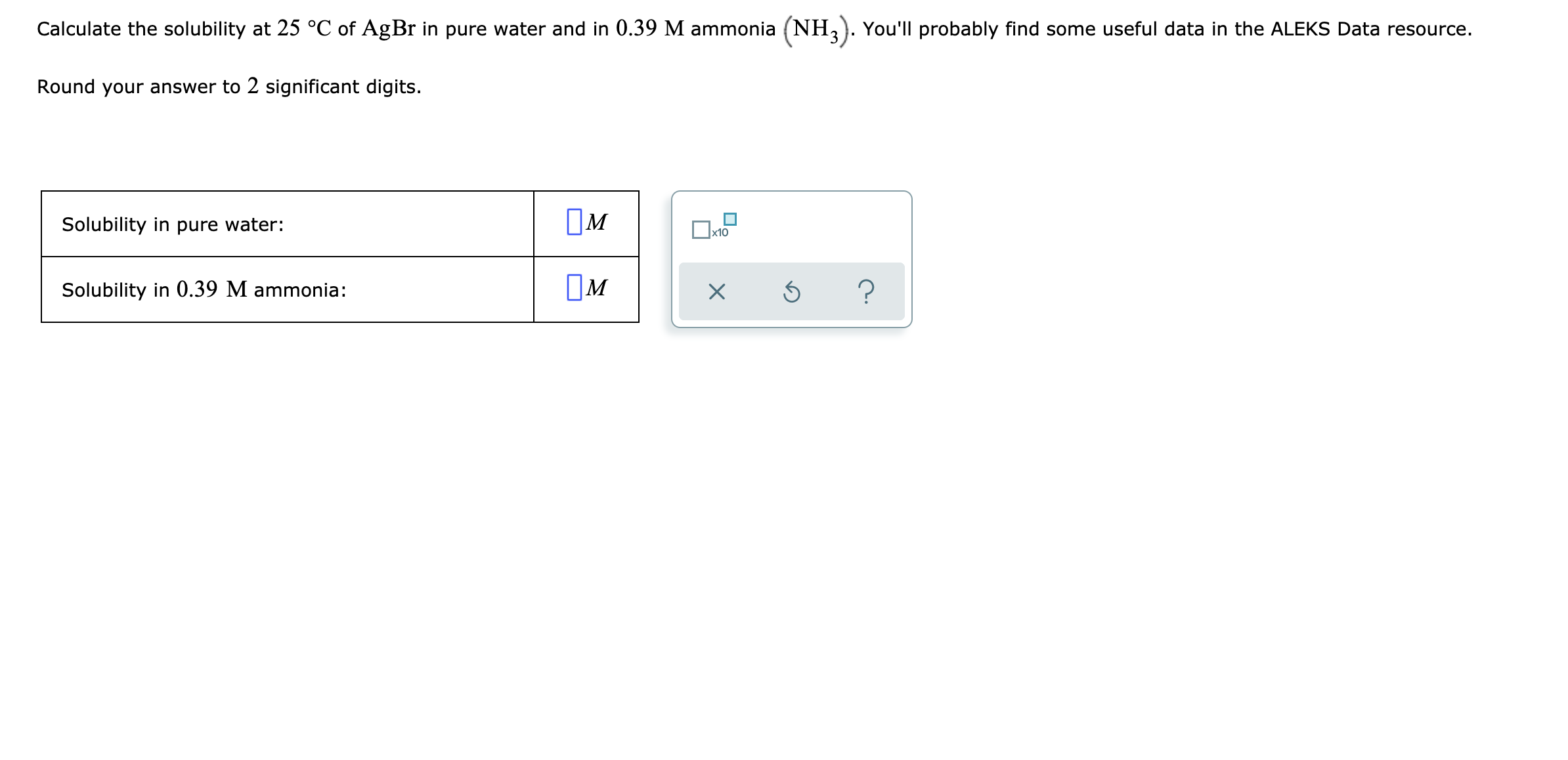
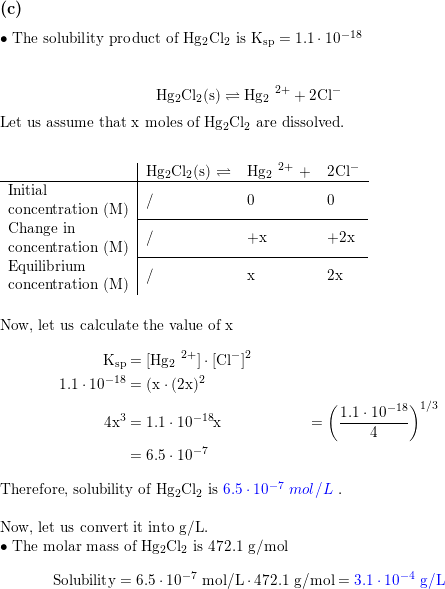![SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH) SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH)](https://cdn.numerade.com/ask_images/7d7ca9e3ac22444fbaeef36ffd903405.jpg)
SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH)

Calculate solubility (in moles / litre) of a saturated aqueous solution of Ag3PO4 if the vapour pressure of the solution becomes 750 torr at 373 K(Assume molality = molarity).
![Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download](https://images.slideplayer.com/27/9060864/slides/slide_2.jpg)
Lesson 4 Calculating Solubility. 1.Calculate the 25 o C for BaCrO 4 in units of g/L. BaCrO 4(s) ⇌ Ba 2+ +CrO 4 2- ssssss Ksp=[Ba 2+ ][CrO. - ppt download
![Calculate solubility of AgCN (K(sp)= 4 xx 10^(-16)) in a buffer solution of PH=3. [(Ka)(HCN)= 4 xx 10^(-10)]. Calculate solubility of AgCN (K(sp)= 4 xx 10^(-16)) in a buffer solution of PH=3. [(Ka)(HCN)= 4 xx 10^(-10)].](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/11037183_web.png)
Calculate solubility of AgCN (K(sp)= 4 xx 10^(-16)) in a buffer solution of PH=3. [(Ka)(HCN)= 4 xx 10^(-10)].